[Introduction]
Azacitidine and venetoclax combination therapy (Aza/Ven) is a novel strategy for acute myeloid leukemia (AML). Although this regimen is widely used as a first line treatment not only for unfit/elderly patients but also for fit/young patients in the real-world situation, appropriate patient selection is an important issue. HM-SCREEN-Japan 02 (UMIN-CTR UMIN000046371) is a Japanese multicenter prospective observational study to evaluate the clinical utility of targeted sequencing. In this study, we performed the sub-analysis of newly diagnosed AML patients who received Aza/Ven as a 1 st line treatment to elucidate the genetic background of AML. Furthermore, we proposed a novel mathematical model to predict the response of Aza/Ven.
[Methods]
AmoyDx® Myeloid Blood Cancer Panel for acute myeloid leukemia was used for the targeted sequencing. We performed linear discriminant analysis to predict complete response patients by Aza/Ven based on clinical information before treatment. We utilized three clinical quantities for the analysis: Wilms Tumor-1 (WT1) mRNA expression level, the number of “favorable mutations”, and that of “unfavorable mutations”. Favorable and unfavorable mutations were defined as IDH1, IDH2, NPM1, and CEBPA mutations, and ASXL1, BCOR, EZH2, RUNX1, SF3B1, SRSF2, STAG2, U2AF1, ZRSR2 and TP53 mutations, respectively, based on the previous reports (NEJM 2019, Blood 2022). The data were standardized and utilized for the linear discriminant analysis. Statistical analyses were performed using R version 4.2.3 software (R Foundation for Statistical Computation, Vienna, Austria).
[Results]
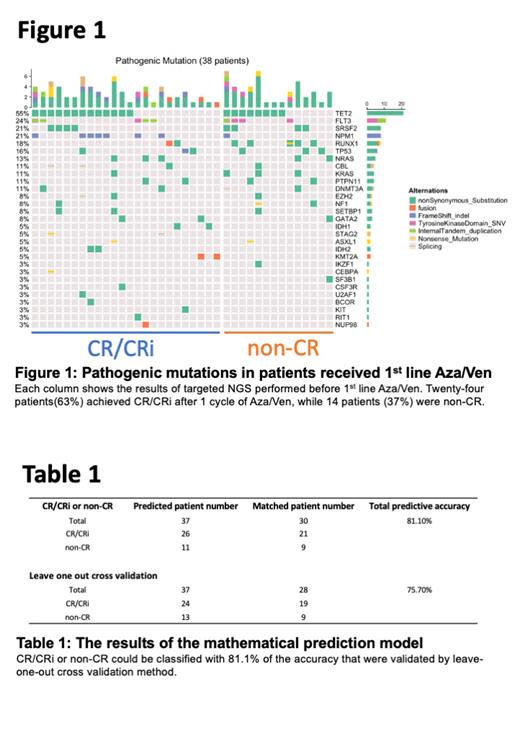
Total 158 patients were enrolled in our study. Among 48 patients who received Aza/Ven as a 1 st line therapy, NGS analyses were performed prior to treatment in 38 patients. Their median age was 70.5 (39-92) years old. AML with myelodysplasia-related changes was the major subtype (71%) and 37 percent of the cases had high risk cytogenetic abnormalities at diagnosis. After 1 st line Aza/Ven treatment, complete remission (CR)/ CR with incomplete blood count recovery (CRi) was achieved in 63% of the cases while the remaining 37% were non-CR. There was a trend that patients with NPM1 and IDH1/2 mutations achieved CR/CRi, while patients with RUNX1 mutation were not tended to achieve CR (Figure 1).
Next, we performed linear discriminant analysis of treatment response using three pretreatment clinical factors. Linear discriminant analysis found a linear combination of prognostic factors that best separates two classes in the group of patients. Thirty-seven patients were employed to determine the discriminant function D, given by D = 0.638 × x − 0.76 × y + 0.68 × z + 0.145. Here x, y, and z represented the WT1 quantity in peripheral blood, the number of “favorable mutations”, and that of “unfavorable mutations” after standardization, respectively. Using this model, 81.1% of cases were correctly classified, and so were 75.7% of cases with leave-one-out cross validation method (Table 1).
[Discussions]
As previously reported (NEJM 2019, Blood adv 2020), IDH1/2 and NPM1 mutations tended to be sensitive to Aza/Ven in our cohort. However, it would be hard to predict the response of Aza/Ven by a single gene mutation because multiple gene mutations were co-existed in most of the cases (Figure 1) and clinical factors including tumor markers might also affect the outcome. Our mathematical model, involving gene mutations and WT1, could efficiently predict the response of Aza/Ven, which may support the selection of 1 st line treatment. In conclusion, our study revealed the genetic landscape of real-world Aza/Ven therapy and provided a potential prognostic model.
Disclosures
Fukushima:Janssen: Honoraria. Hosen:MOCHIDA PHARMACEUTICAL CO.,LTD.: Research Funding; Nippon Shinyaku Co., Ltd.: Research Funding; ONO PHARMACEUTICAL CO., LTD.: Research Funding; Eisai Co., Ltd.: Research Funding; Kyowa Hakko Kirin Co., Ltd.: Research Funding; TEIJIN LIMITED: Research Funding; CHUGAI PHARMACEUTICAL CO., LTD.: Research Funding; Otsuka Pharmaceutical: Research Funding; Shionogi Pharma Co., Ltd.: Research Funding; ASAHI KASEI PHARMA CORPORATION: Research Funding. Yoshimoto:Amgen inc.: Honoraria; Daiichi Sankyo Company, Limited: Honoraria; astellas: Honoraria; AbbVie: Honoraria. Usuki:Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Alnylam Japan: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Alxion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Aperis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Chugai: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Daichi Sankyo: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Eisai: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte Corporation: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kyowa-Kirin: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Mundi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Nippon Shinyaku: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Ohara: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Ono: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Otsuka: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sando: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sumitomo-Dainippon: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; SymBio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Yauklt: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Takahashi:Mochida Pharma: Research Funding; Asahi-Kasei: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Otsuka Pharmacuetical: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Astellas pharma: Other: Commissioned research and joint research , Research Funding. Hosono:Abbvie: Honoraria. Yamauchi:Nihon Kayaku: Honoraria, Research Funding; Sumitomo Pharma: Honoraria, Research Funding; Janssen Pharma: Honoraria, Research Funding; Astellas: Honoraria, Research Funding; Nihon Shinyaku: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; Chugai: Honoraria, Research Funding. Kondo:Pfizer: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Otsuka Pharmaceuticals: Honoraria, Speakers Bureau. Yamamoto:Chugai Pharmaceutical Co., Ltd.: Honoraria; Eisai Co., Ltd.: Honoraria; HUYA/IQIVA: Honoraria; Janssen Pharmaceutical K.K.: Honoraria; Meiji Seika Pharma Co., Ltd.: Honoraria; Molecular Imaging CRO Network/DAIICHI SANKYO COMPANY, LIMITED: Honoraria; Takeda Pharmaceutical: Honoraria; Genmab/IQIVA: Research Funding; Yakult: Research Funding; AbbVie Inc.: Honoraria. Kuroda:Bristol Myeres Squibb, Kyowa Kirin, Chugai, Japan Blood Product Organization, Daiichi Sankyo, Mochida, Ono, Sanofi, Eisai, Taiho, Sumitomo, Asahikasei, Otsuka, Takeda, Shionogi Janssen, Novartis, Abbvie, Pfizer, Nippion Shinyaku, Astellas: Consultancy, Honoraria, Research Funding. Minami:Taiho Pharmaceutical: Research Funding; Takeda: Research Funding; Tejin Pharma: Research Funding; Sumitomo Pharma Oncology: Research Funding; Shionogi: Research Funding; Sanofi: Research Funding; Otsuka: Research Funding; Nippon Shinyaku: Research Funding; Nihonkayaku: Research Funding; Kyowa Hakko Kirin: Research Funding; Dainippon Sumitomo Pharma: Research Funding; Asahi Kasei: Research Funding; Taiho Pharmaceutical: Honoraria; Shinogi: Honoraria; Sanofi: Honoraria; Otsuka: Honoraria; Ono Pharmaceutical: Honoraria; Merck: Honoraria; Meiji Seika Kaisha: Honoraria; Lilly: Honoraria, Research Funding; Kyowa Hakko Kirin: Honoraria; Eisai: Honoraria, Research Funding; Daiichi Sankyo: Honoraria, Research Funding; Chugai Pharma: Honoraria, Research Funding; Bayer: Honoraria, Research Funding; Abbvie: Honoraria; Pfizer Japan Inc.: Honoraria; Bristol-Myers Squibb Company: Honoraria; Takeda: Honoraria; Novartis Pharma KK: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal